National Science Day 2010
- Celebrating the 23rd National Science Day, 28th February, 2010 -
By:- R.K. Birjit Singh *
India is celebrating this day of 28th February as the 'National Science Day' throughout the country with a rich floral tribute to commemorate the great achievement of the discovery of the "Raman Effect" on the 28th February, 1928 by C.V. Raman. In 1986, NCSTC took the initiative to get the Government of India to designate February 28 as the National Science Day. Thereafter, the first National Science Day was observed on the 28th February, 1987.
The basic objective of observation of National Science Day is to spread the message of importance of science and its application among the people. This is essential to accelerate the pace of development. Even in the 21st century and despite many significant achievements certain sections of our society are still guided by blind faith and beliefs, which is reflected in the quality of decision making on developmental issues.
|
Observation of National Science Day attempts at generating scientific minded citizens. Science has contributed a great deal to human welfare. Through the gospel of reason and experimental observation, by which it works, it has enabled man to acquire intellectual and mental excellence. It helps inculcate scientific temper among school children.
Health and hygiene issues are prime concerns for the common people. The daily application of science like the use of clean drinking water, knowledge to eradicate contagious disease, the knowhow of various agricultural practices to increase crop production, the usefulness of biodiversity conservation, etc., should be disseminated to the future generation. Organizing activities with the involvement of large number of people results into purposeful interaction between the science fraternity and the common people for mutual benefit.
Amongst all the scientific discoveries of the modern times, the 'Raman Effect' is perhaps the best known to the Indian public, and for the understandable reasons. But the life of a scientist in India, particularly on those days, was not the life of a gentleman of leisure. It was a perpetual struggle, often bitter, against various odds- some due to the backwardness of the country, some due to the geographic isolation, other arising from diverse hostile forces natural in a cramped and foreign-dominated environment.
And yet Raman, and also many contemporaries of his, did manage to spark the growth of science in India. As we look back one hundred years after his birth, what we perceive in him is not mere great scientist but a microcosm of India with all her problems. In fact, in India he was a rare exception in his passionate involvement with scientific research till the end of his long life.
Chandrasekhar Venkata Raman was born to Chandrasekaran Iyer and Paravti Ammal as their second son on the 7th Novemver,1888 at Tiruvanaikkaval ( At the residence of maternal grandfather) in Tamil Nadu. He had his early education in Madras,(now Chennai). He started his career as an officer in the Finance Ministry but left his job to take up the palit Chair of Physics in Calcutta.
He was professor of physics at the University of Calcutta from 1917 to 1933 and in the latter year was appointed head of the Department of physics of the Indian Institute of Science in Bangalore. In 1947, he became director of the Raman Research Institute, also in Bangalore. He was knighted in 1929 and was named president of the Indian Academy of Sciences in 1934. Raman also studied the physical nature of musical sounds and the mechanics of musical instruments.
He wrote Molecular Diffraction of Light (1922) and The New Physics; Talks on Aspects of Science (1951). For his discovery of Raman Effect, he was awarded the Nobel Prize in physics on the 10th December, 1930 at age of 41. He was the first recipient of Bharat Ratna. He died on 20th November, 1970.
In 1921, When C.V. Raman was traveling by ship from England to India; he was struck by the beautiful hue of the Mediterranean Sea, and began wondering what made it blue. Thus began a chain of enquiry that was to culminate in the discovery of the Raman Effect, whose technological impact continues to felt in this day.
It was known by 1920 that the sky was blue due to a process called Rayleigh scattering. Raman wondered whether the Sea merely reflected the colour of the sky, or had a colour of its own using just a Nicol prism and observing the Sea at a proper angle convinced him that Water itself had a blue color. This led him and his group to undertake a detailed investigation of Rayleigh scattering from solid and liquids. His Experiment led eventually to the discovery of the phenomenon known as "Raman Effect".
"Why the Sky is blue"?
Light is electromagnetic wave. Thus it can affect the motion of the charged particles¬- electrons and atomic nuclei- which are the constituents of all matter. The electron is 2000 times lighter then the lightest of the atomic nuclei. Thus electrons in matter move much more rapidly than the nuclei and over much larger distance. Light falling on matter forces the electrons to oscillated at its own frequency. If this happen to coincide with natural frequency don't match exactly, the electronic motion is affected. The result is that the matter emits light of the same frequency in all directions – light is scattered.
For most materials, the scattering is stronger towards the blue or high frequency end of the spectrum. When sunlight enters the earth's atmosphere, it is scattered by air molecules with blue light being scattered much more strongly than red. This gives the sky its blue color as first argued by Lord Rayleigh. In this explanation, we have neglected the motion of the atomic nuclei. However, the nuclei provide the environment in which electrons move.
Thus the effect of the light on the motion of the electrons is in turn modified (polarized) by the motion of the nuclei. The intensity of the scattered light thus gets modulated with the frequencies associated with the nuclear motions. When the scattered light is analyzed by an instrument called the spectrometer, it shows the original spectral line as the carrier, and satellite spectral lines as sidebands, in much the same way as in the case of radio waves. This phenomenon, first observed by Raman and his group in 1927 is known as "Raman Effect".
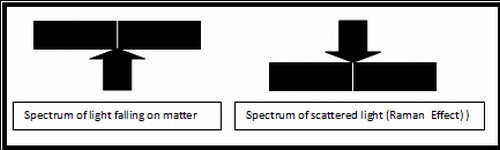
Raman and his co-workers found that of the two new lines, the red shifted was brighter than the blue shifted lines. These are known as Raman Stoke and anti Raman Stoke lines respectively. While this cannot be understood on the basis of the classical description given above, the quantum theory of light gives an adequate explanation of this phenomenon. The knowledge of nuclear motion provides the chemist and technologist an important toll in the study of materials.
The Raman Effect gives an alternative to the improved spectroscopy. It has inherent advantages, as it shifts to spectrum to the visible region where the instrumentation is much more versatile. Moreover, the shorter wavelength allows us to localize the beam much better, permitting small samples. The technological achievement of (importance of Raman Effect in the study of materials has led to the further and more sophisticated experimental set up known as Micro-Raman Analysis, Resonant Raman Analysis, Coherent, Anti-Stokes Analysis and so on.
Raman spectroscopy has widespread recent application in theoretical chemistry. Raman spectra are formed when, under certain conditions, light in the visible or ultraviolet region is first absorbed, and then is re-emitted at a lower frequency after causing molecules to rotate or vibrate.
Two magnetic methods of spectroscopy at the radio-frequency region of the spectrum, longer than the infrared band, are valuable in providing chemical information on molecules and showing their detailed structure. These methods are nuclear-magnetic resonance (nmr) and electron-paramagnetic resonance (epr), the latter also being called electron-spin resonance (esr). These methods depend on the fact that electrons and protons spin like little tops.
To align the spins, the specimen is placed in a magnetic field. Electrons or protons in the specimen "flip" over, reversing their spin axes, when the proper amount of radio-frequency power is supplied.
Thus the nation is giving a rich tribute to the first Asian Novel Laureate in science.
Let the candle of scientific awareness light every home
* R.K. Birjit Singh contributes to e-pao.net regularly . The author is a Science Communicator and can be contacted at bsningthemcha(at)gmail(dot)com
This article was webcasted at e-pao.net on 20th April 2010.
* Comments posted by users in this discussion thread and other parts of this site are opinions of the individuals posting them (whose user ID is displayed alongside) and not the views of e-pao.net. We strongly recommend that users exercise responsibility, sensitivity and caution over language while writing your opinions which will be seen and read by other users. Please read a complete Guideline on using comments on this website.